[email protected]
+91 97269 26402
Manufacturing Process of Manganese Sulphate Monohydrate: A Comprehensive Guide
Manufacturing Process of Manganese Sulphate Monohydrate: A Comprehensive Guide
Hey there, fellow farmers, industry pros, and curious minds! If you’re here to learn about the behind-the-scenes of essential agricultural nutrients, you’ve come to the right place. As someone who’s passionate about helping you make smarter choices for your crops and operations, I want to reassure you that understanding the manufacturing of manganese sulphate can empower you to select the best products. Today, we’re diving into the comprehensive guide on how manganese sulphate monohydrate is made, with insights from trusted processes like those at leading suppliers. Whether you’re a farmer boosting soil health or a manufacturer exploring options, this guide is designed to be clear, supportive, and decision-ready. Let’s explore this together!
What is Manganese Sulphate Monohydrate?
First off, let’s keep it simple. Manganese sulphate monohydrate is a chemical compound with the formula MnSO₄·H₂O. It’s a key micronutrient fertilizer that provides manganese-a vital element for plant growth. Manganese helps with photosynthesis, enzyme activation, and chlorophyll production. Without it, crops like soybeans, wheat, or citrus can suffer from yellowing leaves, stunted growth, and lower yields.
Why focus on monohydrate? It’s the most stable and water-soluble form, making it easy for plants to absorb. As an expert in soil nutrition, I can confidently say that manganese sulphate is indispensable in agriculture, especially in soils deficient in manganese, which is common in alkaline or sandy areas. It’s also used in animal feed, water treatment, and even batteries. If you’re sourcing from a reliable Ferrous Sulphate Heptahydrate Supplier & Manufacturer, chances are they also handle manganese sulphate, offering you a one-stop shop for micronutrients.
The Importance of Manganese Sulphate in Agriculture and Industry
Before we get into the process, let’s talk about why this matters. Manganese is a micronutrient, but its impact is huge. In plants, it supports nitrogen metabolism and protects against oxidative stress. Studies show that manganese-deficient soils can reduce crop yields by up to 20-30%. That’s why applying manganese sulphate granules or solutions is a game-changer for farmers.
Beyond farming, it’s used in industrial applications like dye production and as a catalyst. As a reassuring note, choosing high-quality manganese sulphate ensures safety and effectiveness-always opt for certified suppliers to avoid impurities.
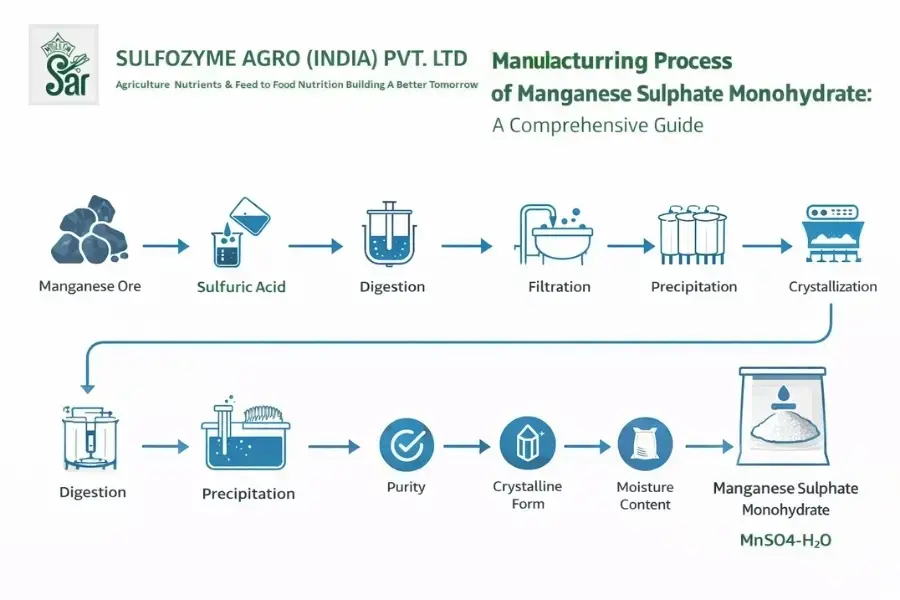
Step-by-Step Manufacturing Process of Manganese Sulphate Monohydrate
Now, the heart of the guide: how is manganese sulphate monohydrate made? I’ll walk you through it like a friendly teacher, breaking it down into clear steps. This process is similar across reputable manufacturers, emphasizing precision and sustainability. Imagine it as transforming raw materials into a nutrient powerhouse.
Step 1: Sourcing Raw Materials – The Foundation
It all starts with manganese ore or manganese dioxide (MnO₂), often sourced from mines. At facilities like those of a top Ferrous Sulphate Heptahydrate Supplier & Manufacturer, we ensure the ore is high-grade, with manganese content around 40-60%. Sulphuric acid (H₂SO₄) is the other key ingredient.
Preparation involves crushing the ore into a fine powder and removing impurities through washing or magnetic separation. This step is crucial for purity-think of it as cleaning the canvas before painting.
Step 2: Chemical Reaction – Dissolving and Reacting
The core reaction happens here. The manganese ore is dissolved in sulphuric acid under controlled conditions. The equation is straightforward: MnO₂ + 2H₂SO₄ → MnSO₄ + 2H₂O + O₂ (with adjustments for monohydrate).
In a reactor, the mixture is heated to 80-120°C, stirred vigorously, and filtered to remove insoluble residues. This produces a manganese sulphate solution. At expert manufacturers, pH and temperature are monitored to prevent side reactions, ensuring a clean, concentrated solution.
Step 3: Purification and Concentration
Next, the solution is purified. Impurities like iron or heavy metals are removed via precipitation or ion exchange. Then, it’s concentrated through evaporation, reducing water content to prepare for crystallization.
This phase is where quality shines-advanced filtration systems ensure the solution is ready for the next step, minimizing waste and maximizing yield.
Step 4: Crystallization – Forming Monohydrate Crystals
Cooling the concentrated solution slowly allows manganese sulphate monohydrate crystals to form. This is done in crystallizers, where temperature is dropped gradually (around 20-30°C). The crystals are then separated via centrifugation or filtration.
Why monohydrate? It has one water molecule, making it stable and easy to handle. Manufacturers like those specializing in manganese sulphate produce crystals that are uniform and pure, often over 98%.
Step 5: Drying, Milling, and Packaging
Quality isn’t optional-it’s essential. Reputable manufacturers conduct tests for manganese content (typically 31-32%), solubility, and contaminants. Techniques include spectroscopy and titration. Safety protocols include ventilation for acid handling and waste neutralization.
Environmentally, the process recycles water and byproducts, aligning with sustainable practices. If you’re a farmer, this means you’re getting a product that’s safe for your soil and ecosystem.
Applications and Benefits of Manganese Sulphate Monohydrate
Let’s tie it back to real-world use. In farming, it’s applied via soil or foliar methods to correct deficiencies. Benefits include improved photosynthesis, disease resistance, and higher yields. For example, in vineyards, it prevents manganese deficiency symptoms.
Industrially, it’s a precursor for other chemicals. As an empathetic guide, I encourage you to consult experts for tailored advice-your success is our priority.
Choosing the Right Supplier
When buying manganese sulphate, look for a Ferrous Sulphate Heptahydrate Supplier & Manufacturer with certifications and positive reviews. They often offer both products, ensuring compatibility and bulk discounts. Prices vary by purity and quantity, but quality pays off.
There you have it-a complete guide to the manufacturing of manganese sulphate monohydrate. From raw ore to finished product, it’s a process of care and precision that supports healthier crops and industries. As a reassuring friend in the field, I’m here to help you navigate this. If you need more details or supplier recommendations, just ask. Let’s grow together!



